Conventionally thought of as a summer phenomenon, ozone pollution has increasingly popped up during wintertime. Over the last ~15-20 years, research has shown that the occurrences are often linked to oil & gas development activities. A new study by Yang et al. investigates the phenomenon of wintertime ozone surges in China and again establishes this link. Their approach to the problem not only reveals some new insights into how and why this happens, and but also suggests some solutions.
The researchers used measurements and modeling to explain the occurrence of four high-ozone episodes in January 2018 in Lanzhou city, in northwestern China. Even though temperatures were below freezing and solar radiation was weak, the hourly ozone values exceeded 100 ppb and were as high as 121 ppb during the episodes. Lanzhou is located in a basin and is one of the largest areas of petrochemical activity in western China–conditions that together are conducive to pollution formation. The design of the study emphasized detailed measurements of volatile organic compounds (VOCs), which proved to be a pivotal choice. An online gas chromatograph-mass spectrometer measured 60 VOCs. In addition, 16 oxygenated VOCs (OVOCs) were measured by collection and subsequent analysis using high-performance liquid chromatography. This treasure-trove of VOC information, along with air quality measurements from the nearby national monitoring site and meteorological measurements from an autonomous weather station, provided the inputs for the photochemical box model/master chemical mechanism used to study the chemistry.
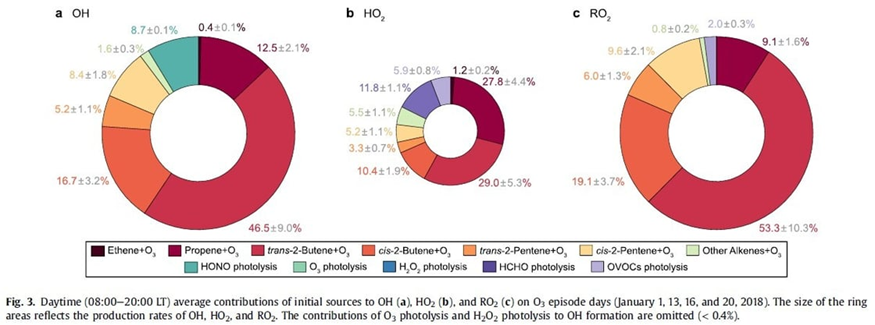
Figure 3 from Yang et al., showing alkene/ozone reactions and other contributions to the formation of reactive radicals OH, HO2, and RO2.
The results showed that alkenes (hydrocarbons with a carbon-carbon double bond) play a large role in the wintertime chemistry that makes ozone pollution. The alkenes react with ozone to produce reactive hydroxyl radicals (OH), which then enter into reaction cycles with the abundant petrochemical VOCs and the nitrogen oxides in the atmosphere to churn out much more ozone than consumed in the initial step. The initial ozone-alkene reactions can proceed under wintertime conditions of low solar and low temperatures. In fact, they can occur in the dark. Yang et al. found that the four least-complicated alkenes accounted for 80% of the ozone production (ethene, propene, trans/cis 2-butene, and trans/cis 2-pentene). The research upends the previous understanding that alkene-ozone reactions mainly lead to ozone destruction. And, this finding of alkene-induced winter ozone pollution suggests a solution: Focusing on reducing alkene (and NOx) emissions could be an effective strategy for mitigating the ozone surges in wintertime.
————————————–
Wintertime Ozone Surges: The Critical Role of Alkene Ozonolysis, J. Yang, Y. Zeren, H. Guo, Y. Wang, X. Lyu, B. Zhou, H. Gao, D. Yao, Z. Wang, S. Zhao, J. Li, and G. Zhang, Environmental Science and Ecotechnology (2024) 22, 100477.

