Researchers from Aerodyne Research Inc., University of Colorado at Boulder, and Pennsylvania State University Investigate the Effect of Varying λ = 185 and 254 nm Photon Flux Ratio on Radical Generation In Oxidation Flow Reactors
Edited from a write-up provided by Andrew Lambe of Aerodyne Research Inc.
The Problem
Oxidation flow reactors (OFRs) complement environmental smog chambers as a portable, low-cost technique for exposing atmospheric compounds to oxidants such as ozone (O3), nitrate (NO3) radicals, and hydroxyl (OH) radicals. OH is most commonly generated in OFRs via photolysis of externally added O3 at λ=254 nm (OFR254) or combined photolysis of O2 and H2O at λ=185 nm plus photolysis of O3 at λ=254 nm (OFR185) using low-pressure mercury (Hg) lamps.
Whereas OFR254 radical generation is influenced by [O3], [H2O], and photon flux at λ=254 nm (I254), OFR185 radical generation is influenced by [O2], [H2O], I185, and I254. Because the ratio of photon fluxes, I185:I254, is OFR-specific, OFR185 performance varies between different systems even when constant [H2O] and I254 are maintained. Thus, calibrations and models developed for one OFR185 system may not be applicable to another.

The Solution
To investigate these issues, we conducted a series of experiments in which I185:I254 emitted by Hg lamps installed in a Potential Aerosol Mass OFR (Aerodyne Research, Inc.) was systematically varied by fusing multiple segments of lamp quartz together that either transmitted or blocked λ=185 nm radiation (Figure 1). Lamp type A is an ozone-producing low-pressure Hg germicidal fluorescent lamp in which type 214 quartz that transmits λ=185 and 254 nm radiation is present along the entire 356 mm arc length. This lamp type is a standard component of the Aerodyne PAM OFR. The relative transmissivity of λ=185 nm radiation (T185) in lamp type A is thus equal to 1. Lamp type B is equivalent to lamp type A with added segments of opaque heat shrink tubing applied to approximately 86 % of the arc length (T185≈0.14) to reduce I185 and I254 to levels below what is achievable using the ballast dimming voltage.
A different type of quartz is available (type 219) that blocks λ=185 nm and transmits λ=254 nm radiation (T185=0). To cover the largest possible range of I185:I254, lamp types C, D, and E fused one segment each of quartz with T185=0 and T185=1 to provide reduced I185 relative to lamp type A while maintaining constant I254. Finally, to evaluate the effect of lamp design at fixed T185 and I254, lamp types F and G contain the same ratios of T185=0 and T185=1 quartz as types C and D, but with 5 and 13 total segments instead of 2 segments. These different designs isolate the effect of discretized λ=185 nm irradiation across the entire arc length of the lamp vs. having all λ=185 nm radiation near the entrance of the OFR. For each lamp type, a fluorescent dimming ballast was used to systematically vary the UV intensity in the reactor.
Radical generation was initiated from photolysis of H2O and O2 at λ = 185 nm and photolysis of O3 at λ = 254 nm according to the reactions:
H2O + hν185 → H + OH
H + O2 → HO2
O2 + hν185 → 2O(3P)
O(3P) + O2 → O3
O3 + hν254 → O2 + O(1D)
O(1D) + H2O → 2OH
Across all experiments, [H2O] ranged from 0.03 % (1 % RH at 25.3 ∘C) to 3.9 % (88 % RH at 30.9 ∘C). The integrated OH exposure (OHexp), defined here as the product of the average OH concentration and the mean OFR residence time (τOFR), was characterized by measuring the decay of carbon monoxide and/or sulfur dioxide tracers. The O3 mixing ratio at the exit of the OFR was measured with a UV ozone analyzer (106-M, 2B Technologies).
The Results
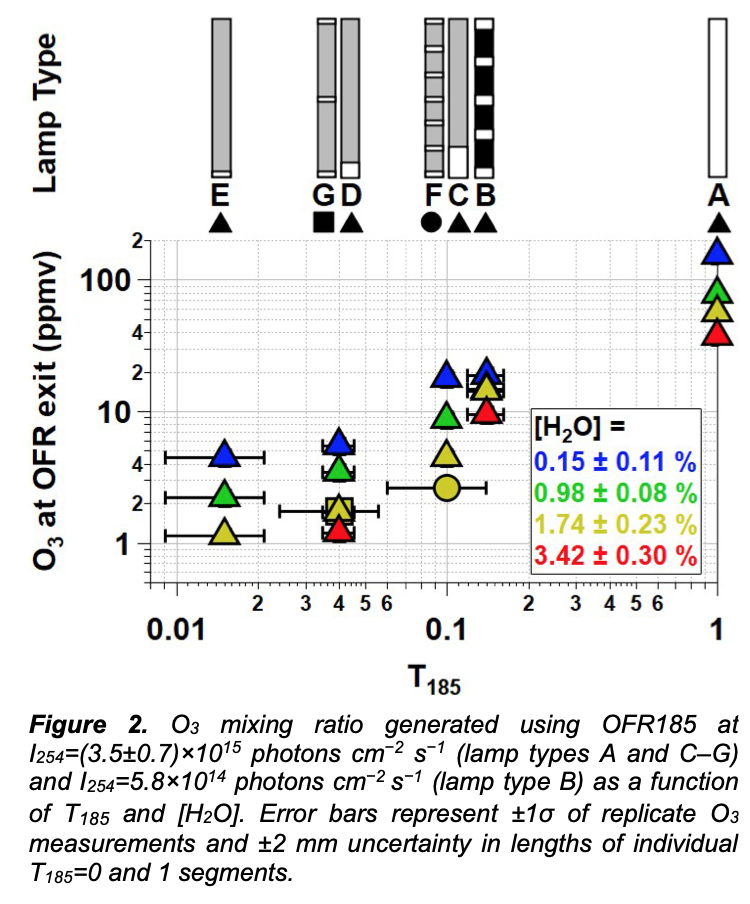
Figure 2 shows [O3] measured at the exit of the OFR as a function of T185 with each lamp type operated at maximum UV output. At fixed [H2O], [O3] increased as a function of T185 due to faster O2 photolysis at λ = 185 nm. At fixed T185 and I254, [O3] decreased with increasing [H2O] due to faster O(1D)+H2O reaction rate following O3 photolysis at λ=254 nm. At [H2O]=1.74 % and T185=0.04 and 0.1, Fig. 2 shows that [O3] generated using lamp types D and G was approximately 1.7 and 1.8 ppmv; here, lamp type D had one 15 mm quartz segment with T185=1, whereas lamp type G had three 5 mm quartz segments with T185=1. Thus, we hypothesize that the OFR- volume-averaged I185 is sufficient to describe associated HOx production for these cases.
Taken together, these measurements were used to develop a set of empirical OHexp estimation equations that parameterized OHexp as a function of OH reactivity, [H2O], [O3], τOFR, and I185:I254. This approach provides a simpler alternative than detailed photochemical models for experimental planning and analysis. Future work will investigate the sensitivity of NOx-dependent, OH-initiated oxygenated volatile organic compound and secondary organic aerosol formation processes to I185:I254.
Click here to view the full paper:
https://acp.copernicus.org/articles/20/13417/2020/
The 2B Tech Instrument’s Role
The Model 106-M was used to measure the ozone mixing ratio at the OFR exit produced by each of the different types of lamps. The instrument’s wide measurement range of 0-1,000 ppm allowed for one instrument to be used to measure the vastly different ozone output from each of the lamps. The high accuracy of the Model 106-M enabled the researchers to be completely confident in reaching their conclusions about [O3] either increasing or decreasing depending on which experimental variables were held constant.
The Bottom Line

If you have an industrial application that requires ozone measurements with high accuracy and precision, then the Model 106-M is right for you. With a measurement range of 0-1,000 ppm the instrument is widely used for university research studies, to measure ozone off-gas in water treatment plants, in food sanitation/cold storage applications, and in various other industrial processes. The instrument can either be provided in a benchtop enclosure for laboratory studies, in an Industrial/NEMA wall-mount enclosure for water treatment and other industrial applications, or as an OEM for integration into an existing system. All versions of the Model 106-M are provided with relays, have various data output options (RS232, USB, 0-2.5 V, 4-20 mA), and have the ability to provide ozone measurements with a fast response time (4 seconds).
Contact 2B Technologies to discuss using the Model 106-M for your application.





